
Resources: National & Regional Guidelines
U.S.A.
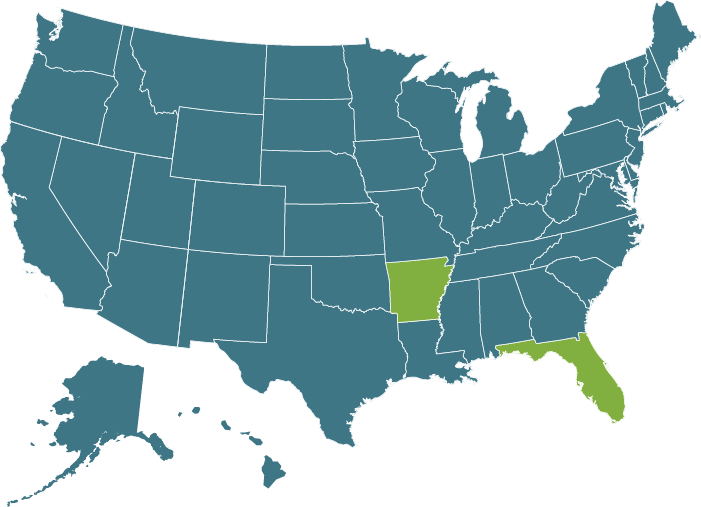
Recommended Testing Frequency
CDC Dental 2003
Correct functioning of sterilization cycles should be verified for each sterilizer by the periodic use (at least weekly) of Biological Indicators
(2,9,134,243,278,279)
*Please note some states have adopted CDC guidelines as law, please refer to your states individual practice act.
ADA State Dental Boards
CANADA
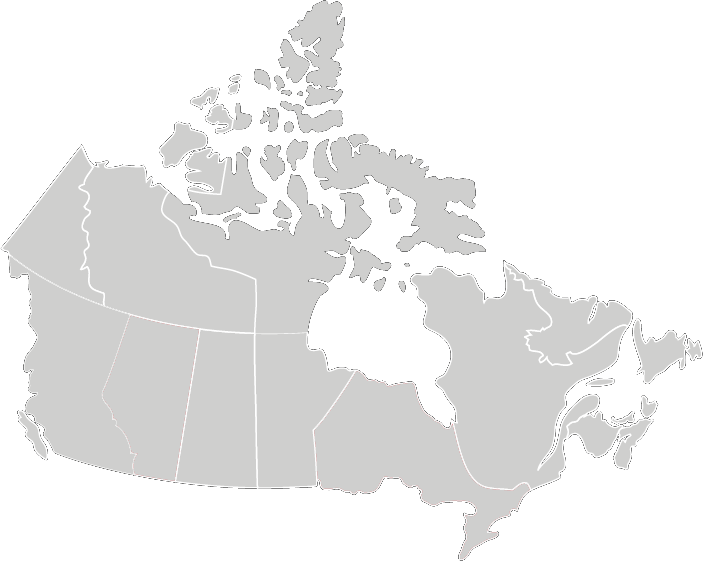
Recommended Testing Frequency
*Note Ontario and Alberta are mandated to test daily
Best Practices for Cleaning, Disinfection and Sterilization of Medical Equipment/Devices
In All Health Care Setting, 3rd Edition May 2013
Provincial Infectious Diseases Advisory Committee (PIDAC)
Biological indicators:
- At least each day that the sterilizer is used
- Each type of cycle that is used that day
- All loads containing implants.

 Recommended Testing Frequency
Recommended Testing Frequency Recommended Testing Frequency
Recommended Testing Frequency
